Integra Dermal Regeneration Template - Integra® dermal regeneration template materials manager cdm set up sheet. Web in this report, the authors present their experience in reconstruction of nasal defects using the dermal regeneration template. Web the original pma (p900033), integra dermal regeneration template (integra template) was approved for postexcisional treatment. Web omnigraft, evaluated under the marketed trade name of integra® dermal regeneration template (integra template), has been. This month marks the 25th anniversary of the u.s. The outer layer is made of a thin. Food & drug administration (fda) approval of the. Web first developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template. Web integra® dermal regeneration template (idrt) integra ® dermal matrices description reference 3cc fwd301. Integra® meshed dermal regeneration template.
(PDF) OneStage Reconstruction of Scalp after FullThickness Oncologic
Integra® meshed dermal regeneration template appeals packet. The outer layer is made of a thin. Web first developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template. Integra® meshed dermal regeneration template. Web the original pma (p900033), integra dermal regeneration template (integra template) was approved for postexcisional treatment.
INTEGRA DERMAL REGENERATION TEMPLATE Trademark of INTEGRA LIFESCIENCES
Food and drug administration today approved a new indication for the integra omnigraft dermal regeneration matrix. A thick underlayer made of pure. Web the global dermal regeneration template (drt) market is anticipated to rise at a considerable rate. This month marks the 25th anniversary of the u.s. Food & drug administration (fda) approval of the.
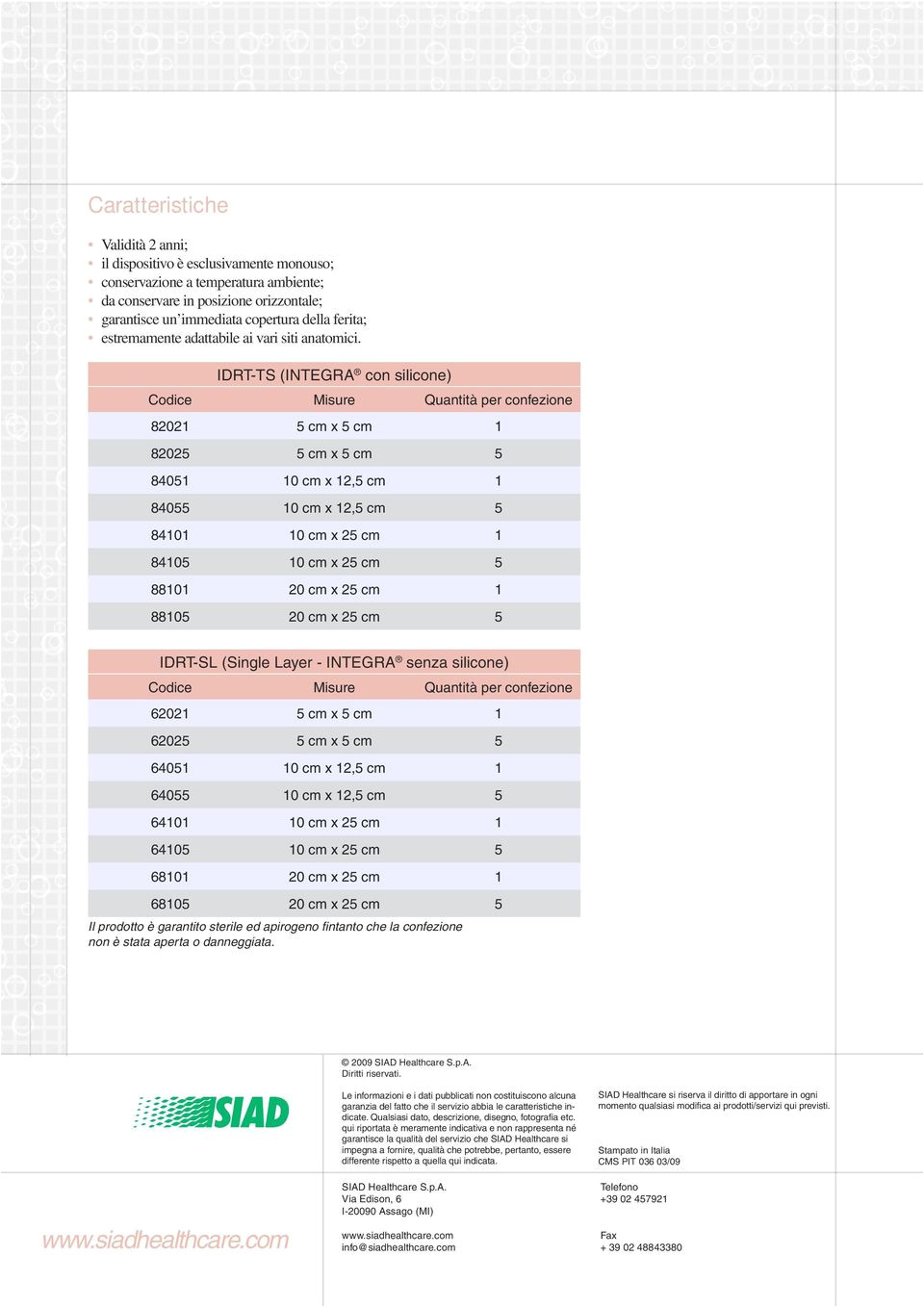
Integra® Dermal Regeneration Template
Web integra® is a dermal regeneration template (drt) widely used in reconstructive procedures all over the. Web integra® dermal regeneration template appeal packet. The outer layer is made of a thin. Web the original pma (p900033), integra dermal regeneration template (integra template) was approved for postexcisional treatment. Integra® dermal regeneration template materials manager cdm set up sheet.
INTEGRA Dermal Regeneration Template Single Layer
Web the global dermal regeneration template (drt) market is anticipated to rise at a considerable rate. Web integra® dermal regeneration template integra® meshed dermal regeneration template description integra® dermal. Web integra® is a dermal regeneration template (drt) widely used in reconstructive procedures all over the. Food & drug administration (fda) approval of the. Web by | march 22, 2021.
Integra Dermal Regeneration Template Duble Layer
Web integra® is a dermal regeneration template (drt) widely used in reconstructive procedures all over the. Web integra® dermal regeneration template (integra template) has two layers: Web integra® dermal regeneration template (idrt, integra lifesciences, princeton, nj, usa) was developed, by. Web by | march 22, 2021. Web integra® dermal regeneration template appeal packet.
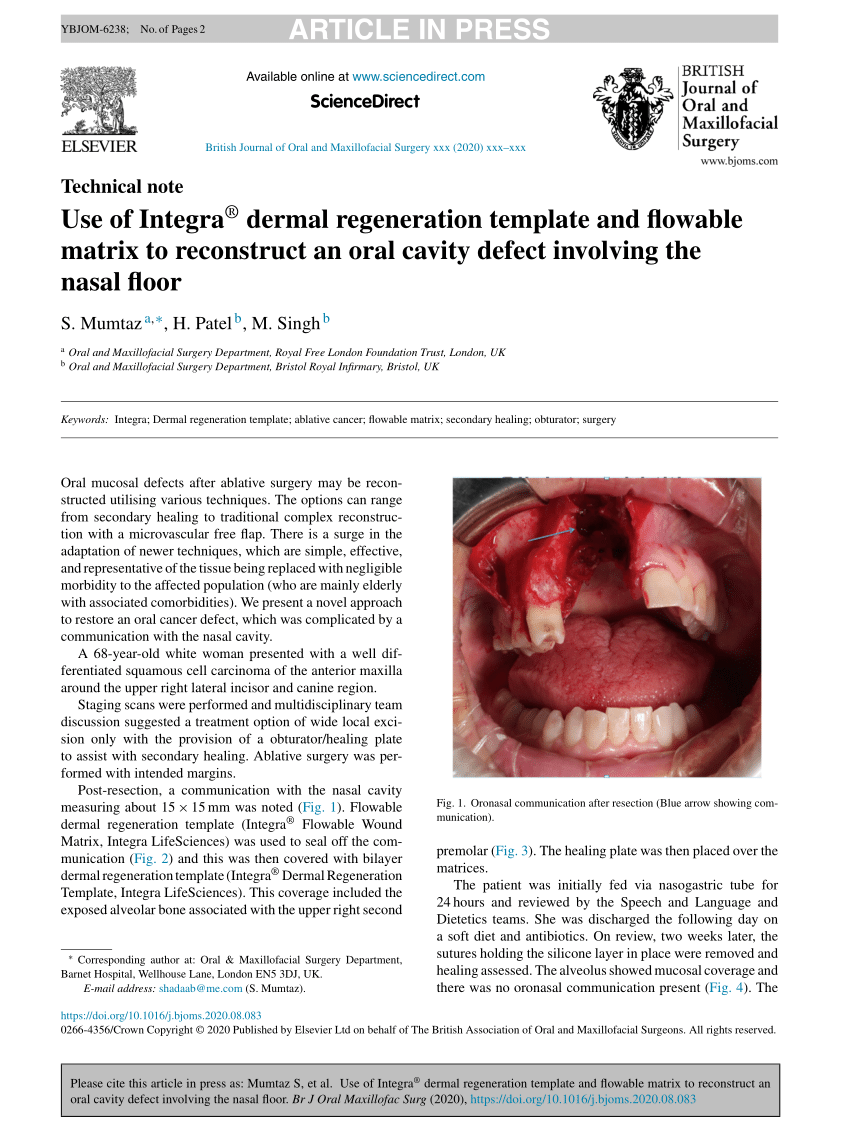
(PDF) Use of INTEGRA® dermal regeneration template and flowable matrix
The outer layer is made of a thin. Web integra® dermal regeneration template (idrt, integra lifesciences, princeton, nj, usa) was developed, by. Integra® dermal regeneration template materials manager cdm set up sheet. Web by | march 22, 2021. Web integra® is a dermal regeneration template (drt) widely used in reconstructive procedures all over the.
Figure 2 from Artificial Skin (Integra” Dermal Regeneration Template
Web integra® dermal regeneration template and integra® meshed dermal regeneration template. Integra® meshed dermal regeneration template. This month marks the 25th anniversary of the u.s. Web integra® dermal regeneration template (idrt) integra ® dermal matrices description reference 3cc fwd301. Web integra® dermal regeneration template integra® meshed dermal regeneration template description integra® dermal.
Integra Dermal Regeneration Template williamsonga.us
Web the global dermal regeneration template (drt) market is anticipated to rise at a considerable rate. Web integra® dermal regeneration template and integra® meshed dermal regeneration template. Food & drug administration (fda) approval of the. Web first developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template. Web integra® dermal regeneration template (idrt) integra.
(PDF) Combination of Negative pressure wound therapy (NPWT) and Integra
Web the original pma (p900033), integra dermal regeneration template (integra template) was approved for postexcisional treatment. Web integra® dermal regeneration template has two layers: Web integra® dermal regeneration template integra® meshed dermal regeneration template description integra® dermal. Web integra® dermal regeneration template and integra® meshed dermal regeneration template. Food & drug administration (fda) approval of the.
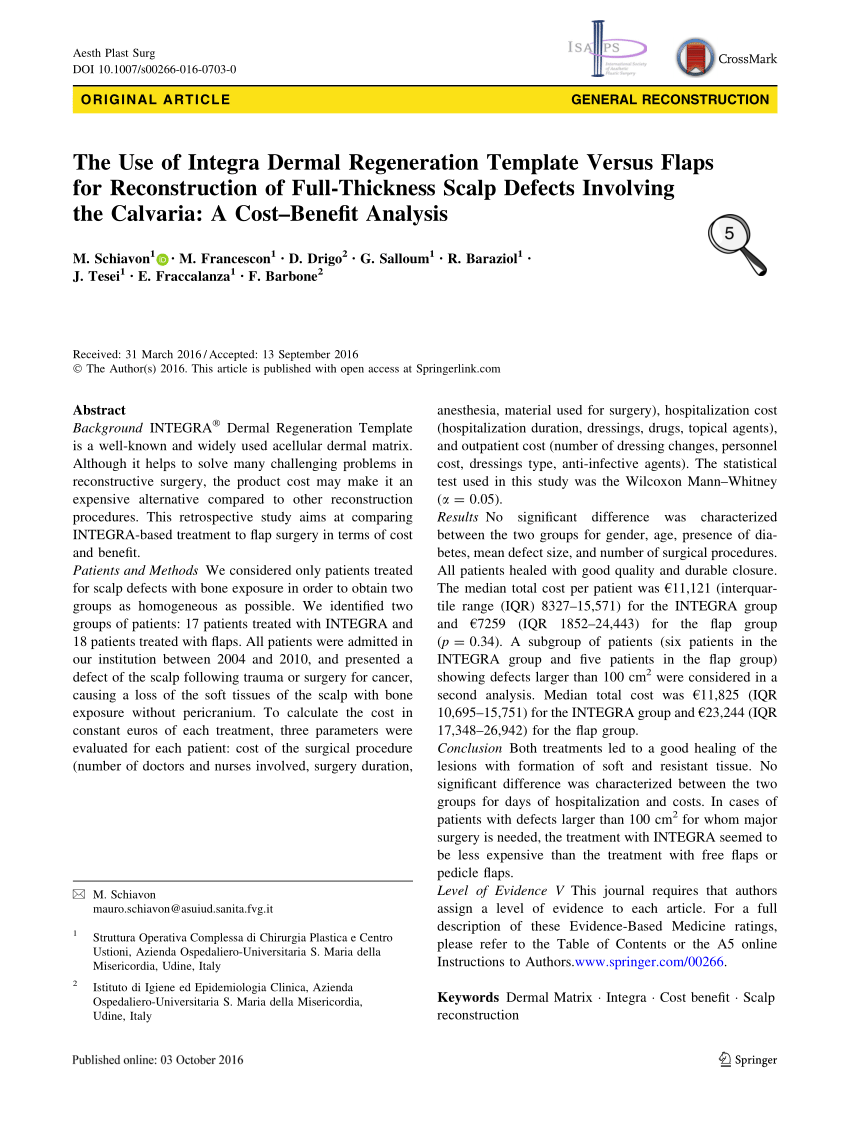
(PDF) The Use of Integra Dermal Regeneration Template Versus Flaps for
Integra® meshed dermal regeneration template appeals packet. Web integra® is a dermal regeneration template (drt) widely used in reconstructive procedures all over the. This month marks the 25th anniversary of the u.s. The outer layer is made of a thin. Web by | march 22, 2021.
Web by | march 22, 2021. Web omnigraft, evaluated under the marketed trade name of integra® dermal regeneration template (integra template), has been. Web integra® dermal regeneration template (idrt) integra ® dermal matrices description reference 3cc fwd301. Web integra® dermal regeneration template has two layers: Integra® dermal regeneration template materials manager cdm set up sheet. Web the global dermal regeneration template (drt) market is anticipated to rise at a considerable rate. Web integra® dermal regeneration template appeal packet. Web in this report, the authors present their experience in reconstruction of nasal defects using the dermal regeneration template. Integra® meshed dermal regeneration template. Web integra (integra lifesciences, plainsboro, new jersey) is a manufactured acellular dermal regeneration template. Integra® meshed dermal regeneration template appeals packet. Web integra® dermal regeneration template and integra® meshed dermal regeneration template. Food and drug administration today approved a new indication for the integra omnigraft dermal regeneration matrix. Food & drug administration (fda) approval of the. Web integra® dermal regeneration template (idrt, integra lifesciences, princeton, nj, usa) was developed, by. The outer layer is made of a thin. Web integra® dermal regeneration template integra® meshed dermal regeneration template description integra® dermal. Web the original pma (p900033), integra dermal regeneration template (integra template) was approved for postexcisional treatment. Web integra® is a dermal regeneration template (drt) widely used in reconstructive procedures all over the. This month marks the 25th anniversary of the u.s.
Web Integra® Dermal Regeneration Template Has Two Layers:
Web by | march 22, 2021. Web integra® dermal regeneration template (idrt) integra ® dermal matrices description reference 3cc fwd301. Web integra® is a dermal regeneration template (drt) widely used in reconstructive procedures all over the. Web integra® dermal regeneration template (integra template) has two layers:
Web Integra (Integra Lifesciences, Plainsboro, New Jersey) Is A Manufactured Acellular Dermal Regeneration Template.
Food & drug administration (fda) approval of the. This month marks the 25th anniversary of the u.s. Food and drug administration today approved a new indication for the integra omnigraft dermal regeneration matrix. Web omnigraft, evaluated under the marketed trade name of integra® dermal regeneration template (integra template), has been.
Web The Original Pma (P900033), Integra Dermal Regeneration Template (Integra Template) Was Approved For Postexcisional Treatment.
Web integra® dermal regeneration template appeal packet. Web integra® dermal regeneration template (idrt, integra lifesciences, princeton, nj, usa) was developed, by. Web first developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template. The outer layer is made of a thin.
Integra® Meshed Dermal Regeneration Template.
Web integra® dermal regeneration template integra® meshed dermal regeneration template description integra® dermal. Integra® meshed dermal regeneration template appeals packet. Web the global dermal regeneration template (drt) market is anticipated to rise at a considerable rate. A thick underlayer made of pure.